Publications

Dinitrogen Binding and Silylation by Bis(ketimino)carbazolide Complexes of Iron and Cobalt
Jack H. Lin, Alexis K. Bauer, Samuel T. Hugie, Michael L. Neidig, Datong Song
Inorg. Chem. 2025, 64, 28, 14566–14576

Coupling reactions using iron–sodium pair - News and Views
Jos Briggs-Pritchard and Michael L. Neidig
Nat. Synth, 2025
DOI: 10.1038/s44160-025-00779-7

Effect of pretreatment conditions on Fe-ZSM-5 properties and performance for Fischer–Tropsch synthesis
Jane N. Agwara, Denis Leshchev, Sinhara M. H. D. Perera, Alexis K. Bauer, Michael L. Neidig and Marc D. Porosoff
Catal. Sci. Technol., 2025,15, 435-447
DOI: 10.1039/D4CY00765D

Synthesis, and Structural and Spectroscopic Analysis of Trielyl-Derived Complexes of Iron
Liam P. Griffin, Alexis K. Bauer, Dr. Agamemnon E. Crumpton, Dr. Mathias A. Ellwanger, Dr. Andreas Heilmann, Dr. Anja Wiesner, Prof. Michael L. Neidig, Prof. Simon Aldridge
Chem. Eur. J. 2025, 31, e202404451

Vanadium Substitution Dictates H Atom Uptake at Lindqvist-type Polyoxotungstates
Dominic Shiels, Zhou Lu, Magda Pascual-Borràs, Nathalia Cajiao, Thompson V. Marinho, William W. Brennessel, Michael L. Neidig, R. John Errington, Ellen M. Matson
Inorg. Chem., 2024, 63, 23304–23316
DOI: 10.1021/acs.inorgchem.4c03873

Activation and Functionalization of the Uranyl Ion by Electrochemical Reduction
Riddhi R. Golwankar, Małgorzata Z. Makoś, Nathalia Cajiao, Michael L. Neidig, Allen G. Oliver, Cynthia S. Day, Victor W. Day, Vassiliki-Alexandra Glezakou, James D. Blakemore#
Inorg. Chem., 2024, 63, 24542–24553
DOI: 10.1021/acs.inorgchem.4c03349

Effective Alkyl-Alkyl Cross-Coupling with an Iron-Xantphos Catalyst: Mechanistic and Structural Insights
Magali Gimeno, Maria Camila Aguilera, Valerie Fleischauer,William Brennessel, and Michael Neidig
Angew. Chem. Int. Ed. 2024, e202413566

Unusual S = 1 Four-Coordinate Fe(IV) Complexes Supported by Bisamide Ligands: Syntheses, Characterization, and Electronic Structures
Zhang B, Joyce JP, Wolford N, Brennessel WW, DeBeer S, Neidig M
Angew. Chem. Int. Ed., 2024, e202405113

Ni(2,2':6',2''-terpyridine)2: a high-spin octahedral formal Ni(0) complex
Cabrera-Lobera N, Del Horno E, Quirós MT, Buñuel E, Gimeno M, Brennessel WW, Neidig ML, Priego JL, Cárdenas DJ
Dalton Trans., 2024, 53, 8550-8554
DOI: 10.1039/d3dt04247b

Mechanistic investigations of the Fe(II) mediated synthesis of squaraines
Liu, Y., Coles, N. T., Cajiao, N., Taylor, L. J.., Davies, E. S.; Barbour, A., Morgan, P., Butler, K.; Pointer-Gleadhill, B., Argent, S., McMaster, J., Neidig, M. L., Robinson, D., Kays, D. L.
Chem. Sci., 2024, 15, 9599-9611
DOI: 10.1039/d4sc01286k

Academia or Industry: Lessons on Choosing Career Paths─There May Be More Than One Fork in the Road Ahead
Blackmond DG, Emmert M, Huryn DM, Neidig ML, Schaub T, Topczewski JJ, Bravo-Altamirano K, Buchan Z, Cabrera PJ
Org. Lett., 2024, 26, 14, 2682–2685

Iron-catalyzed stereoselective C–H alkylation for simultaneous construction of C–N axial and C-central chirality
Zi-Jing Zhang, Nicolas Jacob, Shilpa Bhatia, Philipp Boos, Xinran Chen, Joshua C. DeMuth, Antonis M. Messinis, Becky Bongsuiru Jei, João C. A. Oliveira, Aleksa Radović, Michael L. Neidig, Joanna Wencel-Delord & Lutz Ackermann
Nature Communications, 2024, volume 15, 3503

Caught in the Act of Substitution: Interadsorbate Effects on an Atomically Precise Fe/Co/Se Nanocluster
Kephart JA, Zhou DY, Sandwisch J, Cajiao N, Krajewski SM, Malinowski P, Chu JH, Neidig ML, Kaminsky W, Velian A
ACS Cent. Sci. 2024
DOI: 10.1021/acscentsci.4c00210

Divergent Fe-Mediated C–H Activation Paths Driven by Alkali Cations
Vincent Wowk, Alexis K. Bauer, Aleksa Radovic, Lise-Marie Chamoreau, Michael L. Neidig, and Guillaume Lefèvre
JACS Au, 2024, 4, 2, 512–524
DOI: 10.1021/jacsau.3c00649

Mechanistic manifold in a hemoprotein-catalyzed cyclopropanation reaction with diazoketone
Donggeon Nam, John-Paul Bacik, Rahul L. Khade, Maria Camila Aguilera, Yang Wei, Juan D. Villada, Michael L. Neidig, Yong Zhang, Nozomi Ando & Rudi Fasan
Nature Communications, 2023, 14, 7985
DOI: 10.1038/s41467-023-43559-7

Palladium and Iron Cocatalyzed Aerobic Alkene Aminoboration
Gay, B. L., Wang, Y., Bhatt, S., Tarasewicz, A., Cooke, D. J., Milem, E. G., Zhang, B., Gary, J. B., Neidig, M. L., & Hull, K. L.
J. Am. Chem. Soc. 2023, 145, 18939–18947.
DOI: 10.1021/jacs.3c05790

Mechanistic Studies of Iron-PyBOX-Catalyzed Olefin Amino-Oxygenation with Functionalized Hydroxylamines
Radović, A.; Wolford, N. J.; Li, W.; Brennessel, W. W.; Xu, H.; Neidig, M. L.
Organometallics. 2023, 42, 1810–1817.
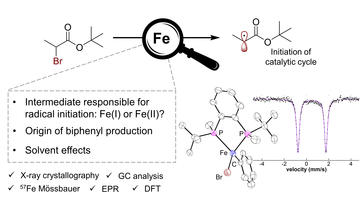
Insight into Radical Initiation, Solvent Effects, and Biphenyl Production in Iron–Bisphosphine Cross-Couplings
Aguilera, M. C.; Gogoi, A. R.; Lee, W.; Liu, L.; Brennessel, W. W.; Gutierrez, O.; Neidig, M. L.
ACS Catal. 2023, 13, 8987–8996.
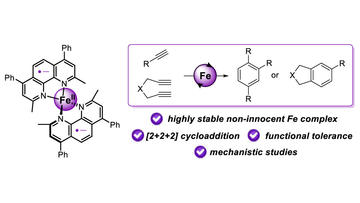
Thermally Stable Redox Noninnocent Bathocuproine-Iron Complex for Cycloaddition Reactions
Féo, M.; Bakas, N. J.; Radović, A.; Parisot, W.; Clisson, A.; Chamoreau, L.; Mansour, Ratovelomanana-Vidal, V.,;Neidig, M. L.; Lefèvre, G.
ACS Catal. 2023, 13, 4882–4893.
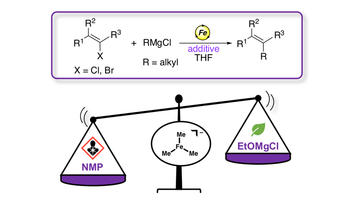
The molecular-level effect of alkoxide additives in iron-catalyzed Kumada cross-coupling with simple ferric salts
Bakas, N. J.; Chourreu P.; Gayon, E.; Lefèvre, G.; Neidig, M. L.
Chem. Commun. 2023, 59, 1317-1320
DOI: 10.1039/D2CC06257G
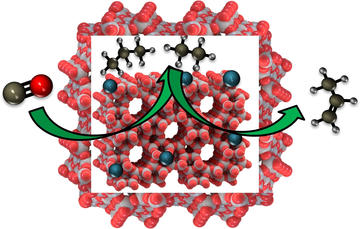
Challenges and Opportunities of Fe-based Core-Shell Catalysts for Fischer-Tropsch Synthesis
Agwara, J. N.; Bakas, N. J.; Neidig, M. L.; & Porosoff, M. D.
ChemCatChem 2022, 14, e202200289.
Homoleptic Uranium–Bis(acyl)phosphide Complexes
Carpenter, S. H.; Wolford, N. J.; Billow, B. S.; Fetrow, T. V.; Cajiao, N.; Radović, A.; Janicke, M. T.; Neidig, M. L.; & Tondreau, A. M.
Inorg. Chem. 2022, 61, 12508–12517.

Anion-induced disproportionation of Th(III) complexes to form Th(II) and Th(IV) products
Wedal, J. C.; Cajiao, N.; Neidig, M. L.; & Evans, W. J.
Chem. Commun. 2022, 58, 5289-5291.
DOI: 10.1039/D2CC01272C
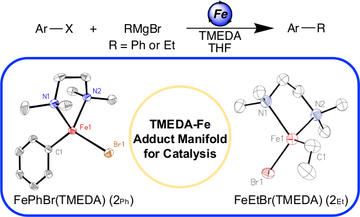
A TMEDA–Iron Adduct Reaction Manifold in Iron-Catalyzed C(sp2)−C(sp3) Cross-Coupling Reactions
Bakas, N. J.; Sears, J. D.; Brennessel, W. W.; Neidig M. L.
Angew. Chem. Int. Ed. 2022, 61, e202114986

Recent Advances in Synthesis, Characterization and Reactivities of Iron-Alkyl and Iron-Aryl Complexes
Zhang, B.; Aguilera, M.C.; Cajiao, N.; Neidig, M.L.
Comprehensive Organometallic Chemistry IV: Volume 1-15. 2022, 185–209

Characterization Methods for Paramagnetic Organometallic Complexes
Radović, A.; Bhatia, S.; Neidig, M.L.
Comprehensive Organometallic Chemistry IV: Volume 1-15. 2022, 135–175

Intermediates and mechanism in iron-catalyzed C–H methylation with trimethylaluminum
Bathia, S.; DeMuth, J.C.; Neidig M. L.
Chem. Commun. 2021, 57, 12784-12787
DOI: 10.1039/D1CC05607G

Creation of an unexpected plane of enhanced covalency in cerium(III) and berkelium(III) terpyridyl complexes
Gaiser, A. N.; Celis-Barros, C.; White, F. D.; Beltrán-Leiva, M. J.; Sperling, J.; Salpage, S. R.; Poe, T. N.; Martinez, D. G.; Jian, T., Wolford, N. J.; Neidig, M. L., & Albrecht‐Schönzart, T. E.; et al.
Nat Commun. 2021, 12, 7230
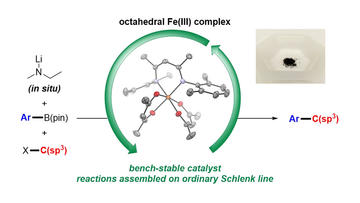
Air-Stable Iron-Based Precatalysts for Suzuki–Miyaura Cross-Coupling Reactions between Alkyl Halides and Aryl Boronic Esters
Wong, A. K. K.; Zhang, B.; Li, B.; Neidig, M. L.; & Byers, J. A.
Org. Process Res. Dev. 2021, 25, 2461–2472

General method for iron-catalyzed multicomponent radical cascades–cross-couplings
Liu, L.; Aguilera, M. C.; Lee, W.; Youshaw, C. R.; Neidig, M. L.; Gutierrez, O.
Science 2021, 374, 432–439
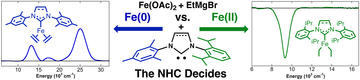
NHC Effects on Reduction Dynamics in Iron-Catalyzed Organic Transformations
Wolford, N. J.; Muñoz, S. B.; Neate, P. G. N.; Brennessel, W. W.; Neidig, M. L.
Chem. Eur. J. 2021, 27, 13651–13658
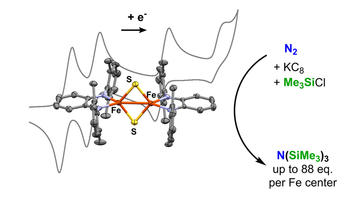
Cluster Supported by Redox-Active o-Phenylenediamide Ligands and Its Application toward Dinitrogen Reduction
Liang, Q.; DeMuth, J.; Radović, A.; Wolford, N. J.; Neidig, M. L.; & Song, D.
Inorg. Chem. 2021, 60, 13811–13820

An anionic iron-hydride superstar for the isomerization of terminal alkenes
Aguilera, M.C.; Neidig, M.L.
Chem Catal. 2021, 1, 488–489

Dilithium Amides as a Modular Bis-Anionic Ligand Platform for Iron-Catalyzed Cross-Coupling
Neate, P. G. N.; Zhang, B.; Conforti, J.; Brennessel, W. W.; Neidig, M. L.
Org. Lett. 2021, 23, 5958–5963

Metal-carbon bonds of iron and manganese
Neidig, M.L., Bakas, N.J., Neate, P.G.N., Sears, J.D.
Comprehensive Coordination Chemistry III. 2021, 82–122

C-H activation/functionalization with earth abundant 3d transition metals
Neidig, M.L., DeMuth, J.C., Zhang, B.
Comprehensive Coordination Chemistry III. 2021, 260–310
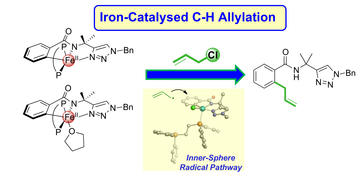
Experimental and computational studies of the mechanism of iron-catalysed C-H activation/functionalisation with allyl electrophiles
DeMuth, J. C.; Song, Z.; Carpenter, S. H.; Boddie, T. E.; Radović, A.; Baker, T. M.; Gutierrez, O.; Neidig, M. L.
Chem. Sci. 2021, 12, 9398–9407
DOI: 10.1039/D1SC01661J
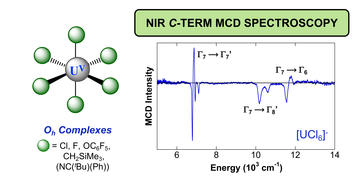
Near-infrared C-term MCD spectroscopy of octahedral uranium(v) complexes
Curran, D. J.; Ganguly, G.; Heit, Y. N.; Wolford, N. J.; Minasian, S. G.; Löble, M. W.; Cary, S. K.; Kozimor, S. A.; Autschbach, J.; Neidig, M. L.
Dalton Trans. 2021, 50, 5483–5492
DOI: 10.1039/D1DT00513H

Forged in iron
Neate, P. G. N.; Neidig, M. L.
Nat Rev Chem. 2021, 5, 223–224

Activation of ammonia and hydrazine by electron rich Fe(II) complexes supported by a dianionic pentadentate ligand platform through a common terminal Fe(III) amido intermediate
Nurdin, L.; Yang, Y.; Neate, P. G. N.; Piers, W. E.; Maron, L.; Neidig, M. L.; Lin, J.; Gelfand, B. S.
Chem. Sci. 2021,12, 2231-2241
DOI: 10.1039/D0SC06466A

C-Term Magnetic Circular Dichroism (MCD) Spectroscopy in Paramagnetic Transition Metal and f-Element Organometallic Chemistry
Wolford, N. J.; Radovic, A.; Neidig, M. L.
Dalton Trans. 2021, 50, 416–428
DOI: 10.1039/D0DT03730C

Additive and Counterion Effects in Iron-Catalyzed Reactions Relevant to C–C Bond Formation
Bakas, N. J.; Neidig, M. L.
ACS Catal. 2021, 11, 8493–8503

CHAPTER 7: Open Shell Iron Catalysis: Mechanistic Challenges, Approaches and Pitfalls
Neate, P.G.N.; Neidig, M.L.
RSC Catalysis Series. 2021, 231–245
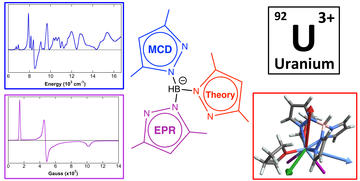
Ligand Effects on Electronic Structure and Bonding in U(III) Coordination Complexes: A Combined MCD, EPR and Computational Study
Wolford, N. J.; Yu, X.; Bart, S. C.; Autschbach, J.; Neidig, M. L.
Dalton Trans. 2020, 49, 14401–14410
DOI: 10.1039/D0DT02929G

Syntheses and characterizations of iron complexes of bulky: O-phenylenediamide ligand
Liang, Q.; Lin, J.H.; Demuth, J.C.; Neidig, M.L.; Song, D.
Dalton Trans. 2020, 49, 12287-12297
DOI: 10.1039/D0DT02087G
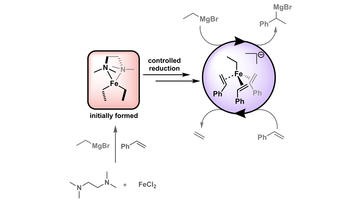
TMEDA in Iron‐Catalyzed Hydromagnesiation: Formation of Iron(II)‐Alkyl Species for Controlled Reduction to Alkene‐Stabilized Iron(0)
Neate, P. G. N.; Greenhalgh, M. D.; Brennessel, W. W.; Thomas, S. P.; Neidig, M. L.
Angew. Chem. Int. Ed. 2020, 59, 17070–17076
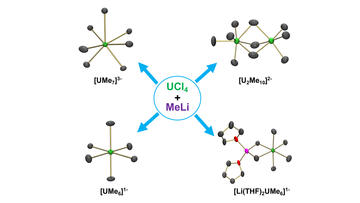
The Exceptional Diversity of Homoleptic Uranium–Methyl Complexes
Sears, J. D.; Sergentu, D.; Baker, T. M.; Brennessel, W. W.; Autschbach, J.; Neidig, M. L.
Angew. Chem. Int. Ed. 2020, 59, 13586–13590
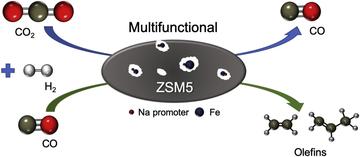
Identifying correlations in Fischer-Tropsch synthesis and CO2 hydrogenation over Fe-based ZSM-5 catalysts
Liu, R.; Ma, Z.; Sears, J. D.; Juneau, M.; Neidig, M. L.; Porosoff, M. D.
J. CO2 Util. 2020, 41, 101290
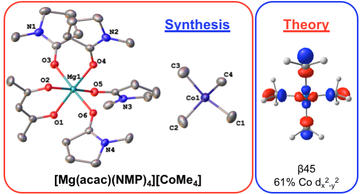
Isolation and Characterization of a Homoleptic Tetramethylcobalt(III) Distorted Square-Planar Complex
Carpenter, S.H.; Brennessel, W.W.; Neidig, M.L.
Organometallics 2019, 38, 3486–3489

Insight into the Electronic Structure of Formal Lanthanide(II) Complexes Using Magnetic Circular Dichroism Spectroscopy
Fleischauer, V. E.; Ganguly, G.; Woen, D. H.; Wolford, N. J.; Evans, W. J.; Autschbach, J.; Neidig, M. L.
Organometallics 2019, 38, 3124–3131
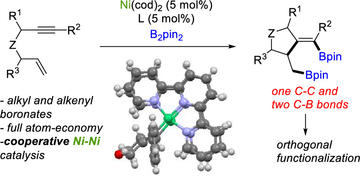
Atom-Economical Ni-Catalyzed Diborylative Cyclization of Enynes: Preparation of Unsymmetrical Diboronates
Cabrera‐Lobera, N., Quirós, M. T., Brennessel, W. W., Neidig, M. L., Buñuel, E., & Cárdenas, D. J.
Org. Lett. 2019, 21, 6552–6556

Identification and Reactivity of Cyclometalated Iron(II) Intermediates in Triazole-Directed Iron-Catalyzed C–H Activation
Boddie, T. E.; Carpenter, S. H.; Baker, T. M.; DeMuth, J. C.; Cera, G.; Brennessel, W. W.; Ackermann, L.; Neidig, M. L.
J. Am. Chem. Soc. 2019, 141, 12338–12345
DOI: 10.1021/jacs.9b05269
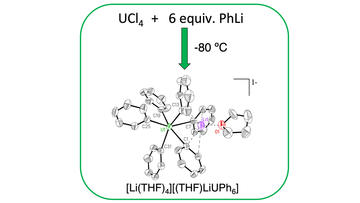
Homoleptic Aryl Complexes of Uranium (IV)
Wolford, N. J.; Sergentu, D.; Brennessel, W. W.; Autschbach, J.; Neidig, M. L.
Chem. Int. Ed. 2019, 58 (30), 10266–10270
Mechanism of the Bis(Imino)Pyridine-Iron-Catalyzed Hydromagnesiation of Styrene Derivatives
Neate, P. G. N.; Greenhalgh, M. D.; Brennessel, W. W.; Thomas, S. P.; Neidig, M. L.
J. Am. Chem. Soc. 2019, 141, 10099–10108
DOI: 10.1021/jacs.9b04869
The Effect of β‐Hydrogen Atoms on Iron Speciation in Cross‐Couplings with Simple Iron Salts and Alkyl Grignard Reagents
Sears, J. D.; Muñoz, S. B.; Daifuku, S. L.; Shaps, A. A.; Carpenter, S. H.; Brennessel, W. W.; Neidig, M. L.
Angew. Chem. Int. Ed. 2019, 58, 2769–2773

Development and Evolution of Mechanistic Understanding in Iron-Catalyzed Cross-Coupling
Neidig, M. L.; Carpenter, S. H.; Curran, D. J.; DeMuth, J. C.; Fleischauer, V. E.; Iannuzzi, T. E.; Neate, P. G. N.; Sears, J. D.; Wolford, N. J.
Acc. Chem. Res. 2019, 52, 140–150
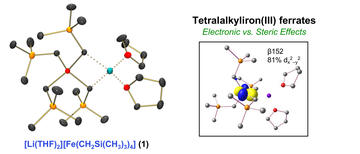
Synthesis and Characterization of a Sterically Encumbered Homoleptic Tetraalkyliron(III) Ferrate Complex
Sears, J. D.; Muñoz, S. B.; Cuenca, M. C. A.; Brennessel, W. W.; Neidig, M. L.
Polyhedron 2019, 158, 91–96

Terminal Coordination of Diatomic Boron Monofluoride to Iron
Drance, M. J.; Sears, J. D.; Mrse, A. M.; Moore, C. E.; Rheingold, A. L.; Neidig, M. L.; Figueroa, J. S.
Science 2019, 363, 1203–1205

Crystal structure of bromidopentakis(tetrahydrofuran-κ O)magnesium bis[1,2-bis(diphenylphosphanyl)benzene-κ2P, P ′]cobaltate(-1) tetrahydrofuran disolvate
Girigiri, P.B.; Carpenter, S.H.; Brennessel, W.W.; Neidig, M.L.
Acta Crystallogr. E: Crystallogr. Commun. 2019, 75, 304–307
Intermediates and Mechanism in Iron-Catalyzed Cross-Coupling
Sears, J. D.; Neate, P. G. N.; Neidig, M. L.
J. Am. Chem. Soc. 2018, 140 (38), 11872–11883
DOI: 10.1021/jacs.8b06893

Combined Effects of Backbone and N-Substituents on Structure, Bonding, and Reactivity of Alkylated Iron(II)-NHCs
Muñoz, S. B.; Fleischauer, V. E.; Brennessel, W. W.; Neidig, M. L.
Organometallics 2018, 37, 3093–3101
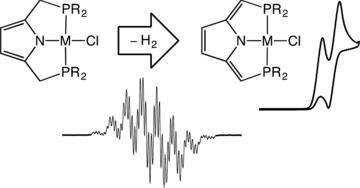
Backbone Dehydrogenation in Pyrrole-Based Pincer Ligands
Krishnan, V. M.; Davis, I.; Baker, T. M.; Curran, D. J.; Arman, H. D.; Neidig, M. L.; Liu, A.; Tonzetich, Z. J.
Inorg. Chem. 2018, 57, 9544–9553
A Pseudotetrahedral Uranium(V) Complex
Tondreau, A. M.; Duignan, T. J.; Stein, B. W.; Fleischauer, V. E.; Autschbach, J.; Batista, E. R.; Boncella, J. M.; Ferrier, M. G.; Kozimor, S. A.; Mocko, V.; Neidig, M. L.; Cary, S. K.; Yang, P.
Inorg. Chem. 2018, 57, 8106–8115
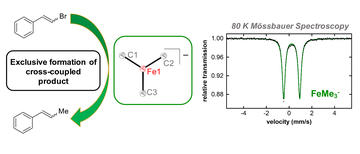
The N-Methylpyrrolidone (NMP) Effect in Iron‐Catalyzed Cross‐Coupling with Simple Ferric Salts and MeMgBr
Muñoz, S. B.; Daifuku, S. L.; Sears, J. D.; Baker, T. M.; Carpenter, S. H.; Brennessel, W. W.; Neidig, M. L.
Chem. Int. Ed. 2018, 57, 6496
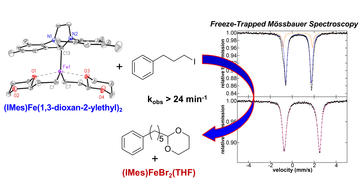
NHC and Nucleophile Chelation Effects on Reactive Iron(II) Species in Alkyl–Alkyl Cross-Coupling
Fleischauer, V. E.; Muñoz III, S. B.; Neate, P. G. N.; Brennessel, W. W.; Neidig, M. L.
Chem. Sci. 2018, 9, 1878–1891
DOI: 10.1039/C7SC04750A

Multinuclear Iron–Phenyl Species in Reactions of Simple Iron Salts with PhMgBr: Identification of Fe4(μ-Ph)6(THF)4
Carpenter, S. H.; Baker, T. M.; Muñoz, S. B.; Brennessel, W. W.; Neidig, M. L.
Chem. Sci. 2018, 9, 7931–7939
DOI: 10.1039/C8SC02915F

Nitric Oxide Activation Facilitated by Cooperative Multimetallic Electron Transfer within an Iron-Functionalized Polyoxovanadate–Alkoxide Cluster
Li, F.; Meyer, R. L.; Carpenter, S. H.; VanGelder, L. E.; Nichols, A. W.; Machan, C. W.; Neidig, M. L.; Matson, E.
Chem. Sci. 2018, 9, 6379–6389
DOI: 10.1039/C8SC00987B

Crystal structures of two new six-coordinate iron(III) complexes with 1,2-bis(diphenylphosphane) ligands
McNeil, D. L., Beckford, D. J., Kneebone, J. L., Carpenter, S. H., Brennessel, W. W., & Neidig, M. L.
Acta Crystallogr. E: Crystallogr. Commun. 2018, 74, 803–807
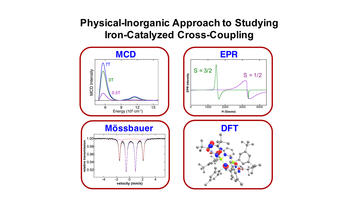
A Physical-Inorganic Approach for the Elucidation of Active Iron Species and Mechanism in Iron-Catalyzed Cross-Coupling
Carpenter, S. H.; Neidig, M. L.
Isr. J. Chem. 2017, 57, 1106–1116

Iron(II) Complexes of a Hemilabile SNS Amido Ligand: Synthesis, Characterization, and Reactivity
Das, U. K.; Daifuku, S. L.; Iannuzzi, T. E.; Gorelsky, S. I.; Korobkov, I.; Gabidullin, B.; Neidig, M. L.; Baker, R. T.
Inorg. Chem. 2017, 56, 13766–13776

A Combined Probe-Molecule, Mössbauer, Nuclear Resonance Vibrational Spectroscopy, and Density Functional Theory Approach for Evaluation of Potential Iron Active Sites in an Oxygen Reduction Reaction Catalyst
Kneebone, J. L.; Daifuku, S. L.; Kehl, J. A.; Wu, G.; Chung, H. T.; Hu, M. Y.; Alp, E. E.; More, K. L.; Zelenay, P.; Holby, E. F.; Neidig, M. L.
J. Phys. Chem. C. 2017, 121, 16283–16290

Polyoxovanadate–Alkoxide Clusters as a Redox Reservoir for Iron
Li, F.; Carpenter, S. H.; Higgins, R. F.; Hitt, M. G.; Brennessel, W. W.; Ferrier, M. G.; Cary, S. K.; Lezama-Pacheco, J. S.; Wright, J. T.; Stein, B. W.; Shores, M. P.; Neidig, M. L.; Kozimor, S. A.; Matson, E. M.
Inorg. Chem. 2017, 56, 7065–7080

Intermediates and Reactivity in Iron-Catalyzed Cross-Couplings of Alkynyl Grignards with Alkyl Halides
Kneebone, J. L.; Brennessel, W. W.; Neidig, M. L.
J. Am. Chem. Soc. 2017, 139, 6988–7003
DOI: 10.1021/jacs.7b02363
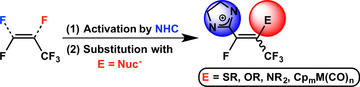
Transition-Metal-Free Formation of C–E Bonds (E = C, N, O, S) and Formation of C–M Bonds (M = Mn, Mo) from N -Heterocyclic Carbene Mediated Fluoroalkene C–F Bond Activation
Leclerc, M. C.; Gabidullin, B. M.; Da Gama, J. G.; Daifuku, S. L.; Iannuzzi, T. E.; Neidig, M. L.; Baker, R. T.
Organometallics 2017, 36, 849–857

Magnetic Circular Dichroism and Density Functional Theory Studies of Electronic Structure and Bonding in Cobalt(II)–N-Heterocyclic Carbene Complexes
Iannuzzi, T. E.; Gao, Y.; Baker, T. M.; Deng, L.; Neidig, M. L.
Dalton Trans. 2017, 46, 13290–13299
DOI: 10.1039/C7DT01748K

Magnetic Circular Dichroism of UCl6− in the Ligand-to-Metal Charge-Transfer Spectral Region
Gendron, F.; Fleischauer, V. E.; Duignan, T. J.; Scott, B. L.; Löble, M. W.; Cary, S. K.; Kozimor, S. A.; Bolvin, H.; Neidig, M. L.; Autschbach, J.
Phys. Chem. Chem. Phys. 2017, 19, 17300–17313
DOI: 10.1039/C7CP02572F

Magnetic Circular Dichroism Studies of Iron(II) Binding to Human Calprotectin
Baker, T. M.; Nakashige, T. G.; Nolan, E. M.; Neidig, M. L.
Chem. Sci. 2017, 8, 1369–1377
DOI: 10.1039/C6SC03487J
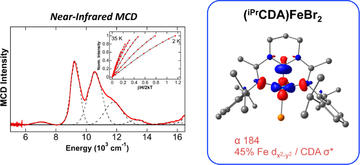
Magnetic Circular Dichroism and Density Functional Theory Studies of Iron(II)-Pincer Complexes: Insight into Electronic Structure and Bonding Effects of Pincer N-Heterocyclic Carbene Moieties
Baker, T. M.; Mako, T. L.; Vasilopoulos, A.; Li, B.; Byers, J. A.; Neidig, M. L.
Organometallics 2016, 35, 3692–3700

Resident Holes and Electrons at Organic/Conductor and Organic/Organic Interfaces: An Electron Paramagnetic Resonance Investigation
Zhang, C.; Daifuku, S. L.; Neidig, M. L.; Marchetti, A. P.
Org. Electron. 2016, 37, 379–385
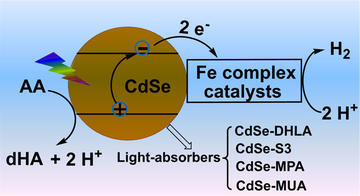
Catalytic Light-Driven Generation of Hydrogen from Water by Iron Dithiolene Complexes
Lv, H.; Ruberu, T. P. A.; Fleischauer, V. E.; Brennessel, W. W.; Neidig, M. L.; Eisenberg, R.
J. Am. Chem. Soc. 2016, 138, 11654–11663
DOI: 10.1021/jacs.6b05040

Manipulating Magneto-Optic Properties of a Chiral Polymer by Doping with Stable Organic Biradicals
Lim, C.-K.; Cho, M. J.; Singh, A.; Li, Q.; Kim, W. J.; Jee, H. S.; Fillman, K. L.; Carpenter, S. H.; Neidig, M. L.; Baev, A.; Swihart, M. T.; Prasad, P. N.
Nano Lett. 2016, 16, 5451–5455
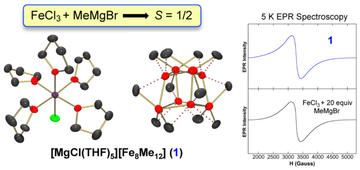
Isolation, Characterization, and Reactivity of Fe8Me12– : Kochi’s S = 1/2 Species in Iron-Catalyzed Cross-Couplings with MeMgBr and Ferric Salts
Muñoz, S. B.; Daifuku, S. L.; Brennessel, W. W.; Neidig, M. L.
J. Am. Chem. Soc. 2016, 138, 7492–7495
DOI: 10.1021/jacs.6b03760

Mononuclear, Dinuclear, and Trinuclear Iron Complexes Featuring a New Monoanionic SNS Thiolate Ligand
Das, U. K.; Daifuku, S. L.; Gorelsky, S. I.; Korobkov, I.; Neidig, M. L.; Le Roy, J. J.; Murugesu, M.; Baker, R. T.
Inorg. Chem. 2016, 55, 987–997

Electronic Structure and Bonding in Iron(II) and Iron(I) Complexes Bearing Bisphosphine Ligands of Relevance to Iron-Catalyzed C–C Cross-Coupling
Kneebone, J. L.; Fleischauer, V. E.; Daifuku, S. L.; Shaps, A. A.; Bailey, J. M.; Iannuzzi, T. E.; Neidig, M. L.
Inorg. Chem. 2016, 55, 272–282

Facile Hydrogen Atom Transfer to Iron(III) Imido Radical Complexes Supported by a Dianionic Pentadentate Ligand
Spasyuk, D. M.; Carpenter, S. H.; Kefalidis, C. E.; Piers, W. E.; Neidig, M. L.; Maron, L.
Chem. Sci. 2016, 7, 5939–5944
DOI: 10.1039/C6SC01433J

Possible Demonstration of a Polaronic Bose-Einstein(-Mott) Condensate in UO2(+x) by Ultrafast THz Spectroscopy and Microwave Dissipation
Conradson, S. D.; Gilbertson, S. M.; Daifuku, S. L.; Kehl, J. A.; Durakiewicz, T.; Andersson, D. A.; Bishop, A. R.; Byler, D. D.; Maldonado, P.; Oppeneer, P. M.; Valdez, J. A.; Neidig, M. L.; Rodriguez, G.
Sci Rep 2015, 5, 15278
DOI: 10.1038/srep15278
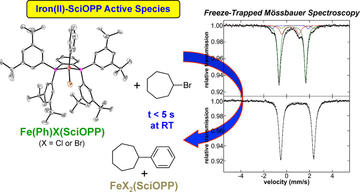
Iron(II) Active Species in Iron–Bisphosphine Catalyzed Kumada and Suzuki–Miyaura Cross-Couplings of Phenyl Nucleophiles and Secondary Alkyl Halides
Daifuku, S. L.; Kneebone, J. L.; Snyder, B. E. R.; Neidig, M. L.
J. Am. Chem. Soc. 2015, 137, 11432–11444
DOI: 10.1021/jacs.5b06648

Linear and T-Shaped Iron(I) Complexes Supported by N-Heterocyclic Carbene Ligands: Synthesis and Structure Characterization
Ouyang, Z.; Du, J.; Wang, L.; Kneebone, J. L.; Neidig, M. L.; Deng, L.
Inorg. Chem. 2015, 54, 8808–8816

Ambivalent Binding between a Radical-Based Pincer Ligand and Iron
Harriman, K. L. M.; Leitch, A. A.; Stoian, S. A.; Habib, F.; Kneebone, J. L.; Gorelsky, S. I.; Korobkov, I.; Desgreniers, S.; Neidig, M. L.; Hill, S.; Murugesu, M.; Brusso, J. L.
Dalton Trans. 2015, 44, 10516–10523
DOI: 10.1039/C5DT01374G

How Innocent Are Potentially Redox Non-Innocent Ligands? Electronic Structure and Metal Oxidation States in Iron-PNN Complexes as a Representative Case Study
Butschke, B.; Fillman, K. L.; Bendikov, T.; Shimon, L. J. W.; Diskin-Posner, Y.; Leitus, G.; Gorelsky, S. I.; Neidig, M. L.; Milstein, D.
Inorg. Chem. 2015, 54, 4909–4926

A Combined Magnetic Circular Dichroism and Density Functional Theory Approach for the Elucidation of Electronic Structure and Bonding in Three- and Four-Coordinate Iron(II)–N-Heterocyclic Carbene Complexes
Fillman, K. L.; Przyojski, J. A.; Al-Afyouni, M. H.; Tonzetich, Z. J.; Neidig, M. L.
Chem. Sci. 2015, 6, 1178–1188
DOI: 10.1039/C4SC02791D

Crystal structure of a third polymorph of tris(acetylacetonato-κ2O,O')iron(III)
Baker, T.M., Howard, K.M., Brennessel, W.W., Neidig, M.L.
Acta Crystallogr. E: Crystallogr. Commun. 2015, 71, m228–m229

Isolation and Characterization of a Tetramethyliron(III) Ferrate: An Intermediate in the Reduction Pathway of Ferric Salts with MeMgBr
Al-Afyouni, M. H.; Fillman, K. L.; Brennessel, W. W.; Neidig, M. L.
J. Am. Chem. Soc. 2014, 136, 15457–15460
DOI: 10.1021/ja5080757

Two- and Three-Coordinate Formal Iron(I) Compounds Featuring Monodentate Aminocarbene Ligands
Mo, Z.; Ouyang, Z.; Wang, L.; Fillman, K. L.; Neidig, M. L.; Deng, L.
Org. Chem. Front. 2014, 1, 1040–1044
DOI: 10.1039/C4QO00175C
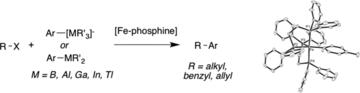
Iron Phosphine Catalyzed Cross-Coupling of Tetraorganoborates and Related Group 13 Nucleophiles with Alkyl Halides
Bedford, R. B.; Brenner, P. B.; Carter, E.; Clifton, J.; Cogswell, P. M.; Gower, N. J.; Haddow, M. F.; Harvey, J. N.; Kehl, J. A.; Murphy, D. M.; Neeve, E. C.; Neidig, M. L.; Nunn, J.; Snyder, B. E. R.; Taylor, J.
Organometallics 2014, 33, 5767–5780
DOI: 10.1021/om500518r

A Combined Mössbauer, Magnetic Circular Dichroism, and Density Functional Theory Approach for Iron Cross-Coupling Catalysis: Electronic Structure, In Situ Formation, and Reactivity of Iron-Mesityl-Bisphosphines
Daifuku, S. L.; Al-Afyouni, M. H.; Snyder, B. E. R.; Kneebone, J. L.; Neidig, M. L.
J. Am. Chem. Soc. 2014, 136 (25), 9132–9143
DOI: 10.1021/ja503596m
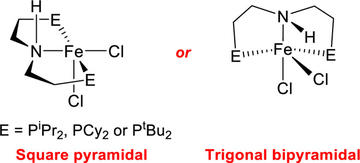
Flexible Binding of PNP Pincer Ligands to Monomeric Iron Complexes
Fillman, K. L.; Bielinski, E. A.; Schmeier, T. J.; Nesvet, J. C.; Woodruff, T. M.; Pan, C. J.; Takase, M. K.; Hazari, N.; Neidig, M. L.
Inorg. Chem. 2014, 53, 6066–6072
DOI: 10.1021/ic5004275

Iron Dicarbonyl Complexes Featuring Bipyridine-Based PNN Pincer Ligands with Short Interpyridine C-C Bond Lengths: Innocent or Non-Innocent Ligand?
Zell, T.; Milko, P.; Fillman, K. L.; Diskin-Posner, Y.; Bendikov, T.; Iron, M. A.; Leitus, G.; Ben-David, Y.; Neidig, M. L.; Milstein, D.
Chem. Eur. J. 2014, 20, 4403–4413
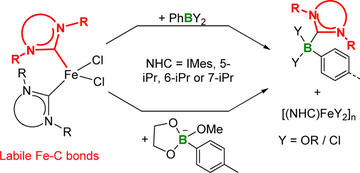
Reactivity of (NHC)2FeX2 Complexes toward Arylborane Lewis Acids and Arylboronates
Dunsford, J. J.; Cade, I. A.; Fillman, K. L.; Neidig, M. L.; Ingleson, M. J.
Organometallics 2014, 33, 370–377
DOI: 10.1021/om401105k
Direct Observation of ICT Cations at the HTL/Transparent Semiconductor Interface
Daifuku, S. L.; Favaro, C.; Marchetti, A. P.; Neidig, M. L.
Org. Chem. Front. 2014, 15, 3761–3765

Efficient Nazarov Cyclization/Wagner-Meerwein Rearrangement Terminated by a CuII-Promoted Oxidation: Synthesis of 4-Alkylidene Cyclopentenones
Lebœuf, D.; Theiste, E.; Gandon, V.; Daifuku, S. L.; Neidig, M. L.; Frontier, A. J.
Chem. Eur. J. 2013, 19, 4842–4848

Covalency in F-Element Complexes
Neidig, M. L.; Clark, D. L.; Martin, R. L.
Coord. Chem. Rev. 2013, 394–406



